Unlocking lignocellulose: how the extreme thermophile Anaerocellum bescii grabs its sugars.
- Ray Sullivan

- May 30, 2025
- 3 min read

Converting renewable plant biomass, or lignocellulose, into valuable fuels and chemicals using microbial processes is an attractive, environmentally friendlier alternative to methods based on fossil fuels. This process, often envisioned as Consolidated Bioprocessing (CBP), ideally involves microbes that can both break down the complex lignocellulose and produce desired products in a single step. However, finding or engineering microbes that are highly efficient at both tasks is challenging.
Enter thermophilic microorganisms, those that thrive at high temperatures. Among these, Anaerocellum bescii, stands out as a promising candidate for lignocellulose deconstruction and conversion. This extremely thermophilic, Gram-positive, anaerobic bacterium has an optimal growth temperature of 78°C. It is known for its ability to effectively degrade both the cellulose and hemicellulose components of lignocellulosic biomass, utilizing a diverse set of secreted and surface-associated multi-domain enzymes. A. bescii has also been genetically engineered to produce industrially relevant chemicals like acetone, hydrogen, and ethanol from lignocellulose.
While there is significant understanding of how A. bescii breaks down plant biomass externally, a critical, yet less explored, step is how the sugars released from this breakdown are transported into the microbial cells for conversion into products. A recent paper by Jonathan Conway’s Lab at Princeton University dives into this crucial aspect, focusing on the transport of maltodextrins in Anaerocellum bescii.
High-affinity ATP-binding cassette (ABC) transporters play a vital role in carbohydrate transport in lignocellulolytic thermophiles like A. bescii. These transporters typically consist of an extracytoplasmic substrate-binding protein, transmembrane proteins, and a cytoplasmic ATPase.
In A. bescii, the maltodextrin ABC transporters belong to the CUT1 family, which typically prefers transporting larger oligosaccharides. Interestingly, they are likely powered by a single, promiscuous ATPase called MsmK (Athe_1803). An A. bescii strain with a deleted msmK gene showed impaired growth on maltose, supporting the idea that MsmK powers these transporters.
Conway’s lab identified two distinct ABC transporters in A. bescii predicted to transport maltodextrins, based on previous transcriptomic studies and genomic analysis: Athe_2308-2310 and Athe_2574-2578. They conducted experiments using purified substrate-binding proteins from these transporters to figure out exactly which sugars they bind.
Their findings revealed an interesting division of labor:
• Athe_2308-2310 strongly interacts with shorter maltodextrins, specifically maltose (a disaccharide) and trehalose, binding them with high affinity (dissociation constants in the micromolar range).
• Athe_2574-2578 preferentially binds longer malto-oligosaccharides, showing affinity for chains larger than maltotriose (a trisaccharide).
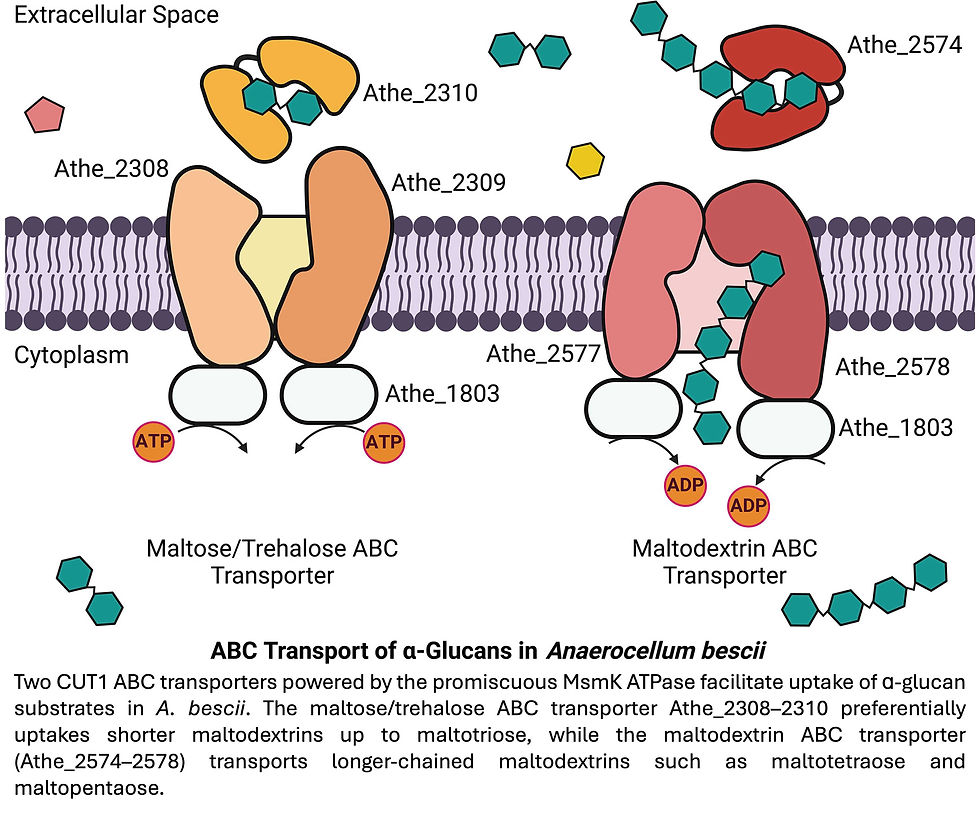
This strategy of using separate transporters for different sugar lengths offers metabolic benefits. Transporting longer oligosaccharides provides a higher return on the ATP energy invested per sugar molecule. Having transporters for both short and long sugars allows A. bescii to efficiently utilize a wider range of carbohydrates available in its environment, mitigating the risk if extracellular enzymes break down complex sugars into various sizes. This contrasts with organisms like E. coli, whose maltodextrin-binding protein binds a broad range of maltodextrin lengths non-selectively.
Their research employed techniques like differential scanning calorimetry (DSC) and isothermal titration calorimetry (ITC) to experimentally determine the binding specificity and affinity of these proteins. By combining these biophysical methods with structural analysis and modeling, the group provided context for the observed differences in binding.
In summary, the work by the Conway Lab and colleagues sheds light on the intricate sugar transport system of A. bescii, demonstrating that it utilizes specific, high-affinity ABC transporters to efficiently uptake maltodextrins of different lengths. This deeper understanding of sugar transport mechanisms is crucial for continuing to engineer A. bescii and similar hyperthermophiles to become more efficient platforms for converting plant biomass into the fuels and chemicals needed for a sustainable bioeconomy.
Tjo H, Jiang V, Joseph JA, Conway JM. Maltodextrin transport in the extremely thermophilic, lignocellulose degrading bacterium Anaerocellum bescii (f. Caldicellulosiruptor bescii). J Bacteriol. 2025 May 22;207(5):e0040124. doi: 10.1128/jb.00401-24. Epub 2025 Apr 30. PMID: 40304524; PMCID: PMC12096829.










Comments