How HSV-1 Hijacks Your Cells' Ribosome Factories
- Ray Sullivan

- Jul 11
- 4 min read

Imagine your cells as bustling cities, meticulously organized with specialized districts (compartments) to keep everything running smoothly. The nucleus, for example, is the city hall where genetic blueprints are managed, while the cytoplasm is the industrial zone where proteins are built. But what happens when a cunning invader like the Herpes Simplex Virus type 1 (HSV-1) infiltrates this city? It doesn't just wreak havoc; it strategically remodels the urban landscape, taking control of vital resources to ensure its own replication and survival.
A new study by Metzger, Reed, et al., led by Ileana Cristea at Princeton, sheds light on one of HSV-1's most sophisticated strategies: hijacking the cell's protein-making machinery, the ribosomes, by creating specialized nuclear aggregates. This research, leveraging an innovative technique called Nuclear/Cytoplasmic Thermal Proximity Coaggregation Analysis (N/C-TPCA), offers insight into the dynamic, spatially resolved protein-protein interactions (PPIs) that underpin this viral takeover.
Understanding how viruses manipulate host cells requires a detailed view of how proteins interact and move within different cellular compartments over time. Traditional methods for studying protein interactions often analyze whole cells, which can obscure crucial localization-dependent PPIs or low-abundance complexes.
This is where N/C-TPCA comes in. The researchers integrated subcellular fractionation (separating the nucleus and cytoplasm) with thermal proximity coaggregation analysis (TPCA). TPCA works on the principle that interacting proteins tend to denature and aggregate together when heated, exhibiting correlated thermal stability profiles. By performing thermal denaturation before fractionation, the method preserves protein interactions, allowing for the precise measurement of PPIs in both the nuclear and cytoplasmic compartments.
Here's a breakdown of the N/C-TPCA method:
TPCA is based on the principle that interacting proteins tend to behave as a unit. When heated, these interacting proteins will denature and aggregate together, displaying correlated thermal solubility profiles. This biochemical property allows TPCA to globally monitor PPIs for endogenous proteins.
Integration with Subcellular Fractionation: Unlike conventional TPCA that analyzes whole-cell samples (which can obscure localization-dependent PPIs or low-abundance complexes), N/C-TPCA adds a subcellular spatial dimension. It involves separating cellular components into nuclear and cytoplasmic fractions.
Experimental Workflow:
Cells are first subjected to thermal denaturation across a range of temperatures (e.g., 37° to 55°C). This step is performed before subcellular fractionation to better maintain protein-protein interactions.
Following thermal denaturation, nuclear/cytoplasmic fractionation is performed. Centrifugation separates the insoluble protein pellets from the soluble proteins in the supernatant for each compartment.
The soluble proteins from both the nuclear and cytoplasmic fractions are then processed through a mass spectrometry (MS) workflow.
Data from the MS analysis are used to predict PPIs, often utilizing machine learning–based frameworks like Tapioca.
N/C-TPCA provides a significantly expanded depth and spatial resolution of PPIs compared to previous whole-cell studies. The enhanced resolution allowed the Cristea group to detect distinct subsets of assembled protein complexes in each compartment, revealing a more holistic picture of how cells are reorganized during infection.
Armed with N/C-TPCA, the group investigated the host-pathogen interactions during HSV-1 infection. They used the ICP0-RF strain, a mutant of HSV-1 that is known to potentiate an intrinsic immune signaling response orders of magnitude higher than wild-type. They found that HSV-1 orchestrates extensive intracellular remodeling, including mechanisms to halt host gene expression and protein translation.
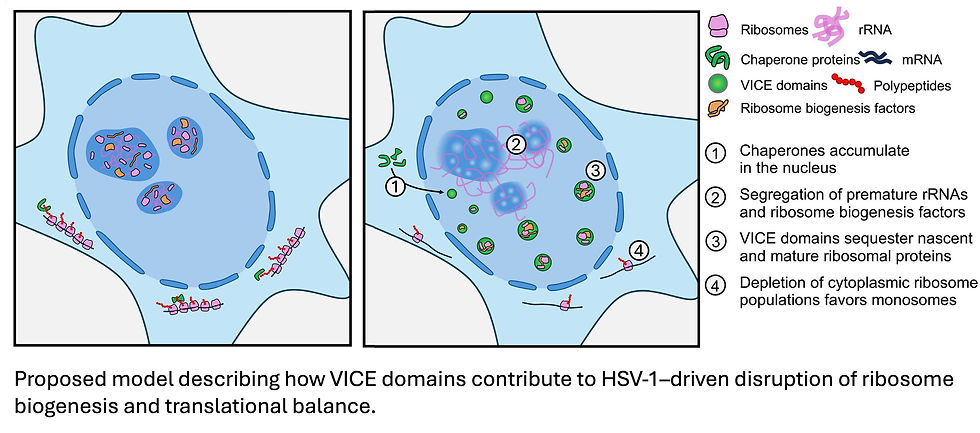
A key discovery centered on Virus-Induced Chaperone-Enriched (VICE) domains. These are nuclear aggregates that form during HSV-1 infection, previously known as sites of nuclear protein quality control. The study found that a broader suite of host chaperones than previously anticipated are recruited to form these VICE domains.
Their research revealed that VICE domains actively sequester ribosome biogenesis factors from ribosomal RNA (rRNA). In healthy cells, these factors and rRNA normally co-localize in the nucleolus to build new ribosomes. However, upon HSV-1 infection, this spatial organization is disrupted. The ribosome biogenesis proteins are trapped within VICE domains, while premature rRNA is excluded. This creates a "spatial decoupling" of critical ribosome-building components.
This sequestration has consequences:
It leads to a cell-wide defect in ribosome supply.
Both small (40S) and large (60S) ribosomal subunits are affected, indicating a problem with their synthesis or stability.
VICE domains recruit both newly translated ribosomal proteins and components of mature ribosomes that existed long before infection, exerting a "sponging effect". This suggests that HSV-1 not only prevents new ribosome production but also draws away existing ribosomal components from the cytoplasm into the nucleus, contributing to the observed loss of polysomes (active protein-making complexes) in the cytoplasm.
These findings fit into the broader picture of how HSV-1 controls the balance between viral and host gene expression. By creating a ribosome-limiting environment, HSV-1 gives its own specialized viral factors, like VP22, a competitive advantage to maintain translation in a manner favorable for viral growth and spread. This explains how HSV-1 can cause translational shutoff in host cells while still managing to produce its own proteins efficiently.
This study not only uncovers fundamental mechanisms of viral infection but also powerfully demonstrates the utility of N/C-TPCA as a tool to investigate dynamic biological processes with unprecedented spatial and temporal specificity. It offers a significant step forward in understanding the intricate warfare between viruses and their hosts at a molecular level.
Metzger PJ, Reed TJ, Lum KK, Botello JF, Jiang L, Brangwynne CP, Troyanskaya OG, Cristea IM. Sequestration of ribosome biogenesis factors in HSV-1 nuclear aggregates revealed by spatially resolved thermal profiling. Sci Adv. 2025 Jun 27;11(26):eadw6814. doi: 10.1126/sciadv.adw6814. Epub 2025 Jun 27. PMID: 40577456; PMCID: PMC12204162.










Comments